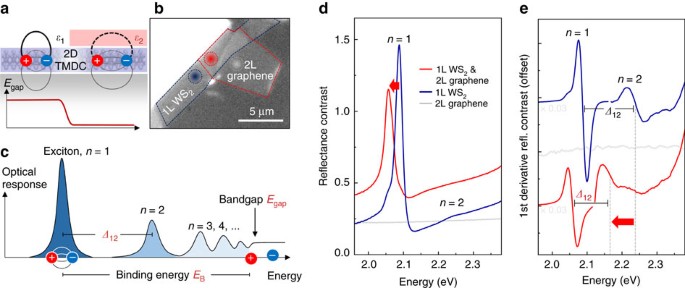
Action Movies In 2020 The Binding Energy Of An Electron In The Ground State In A Hydrogen Atom Is About
Action Movies In 2020 The Binding Energy Of An Electron In The Ground State In A Hydrogen Atom Is About, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
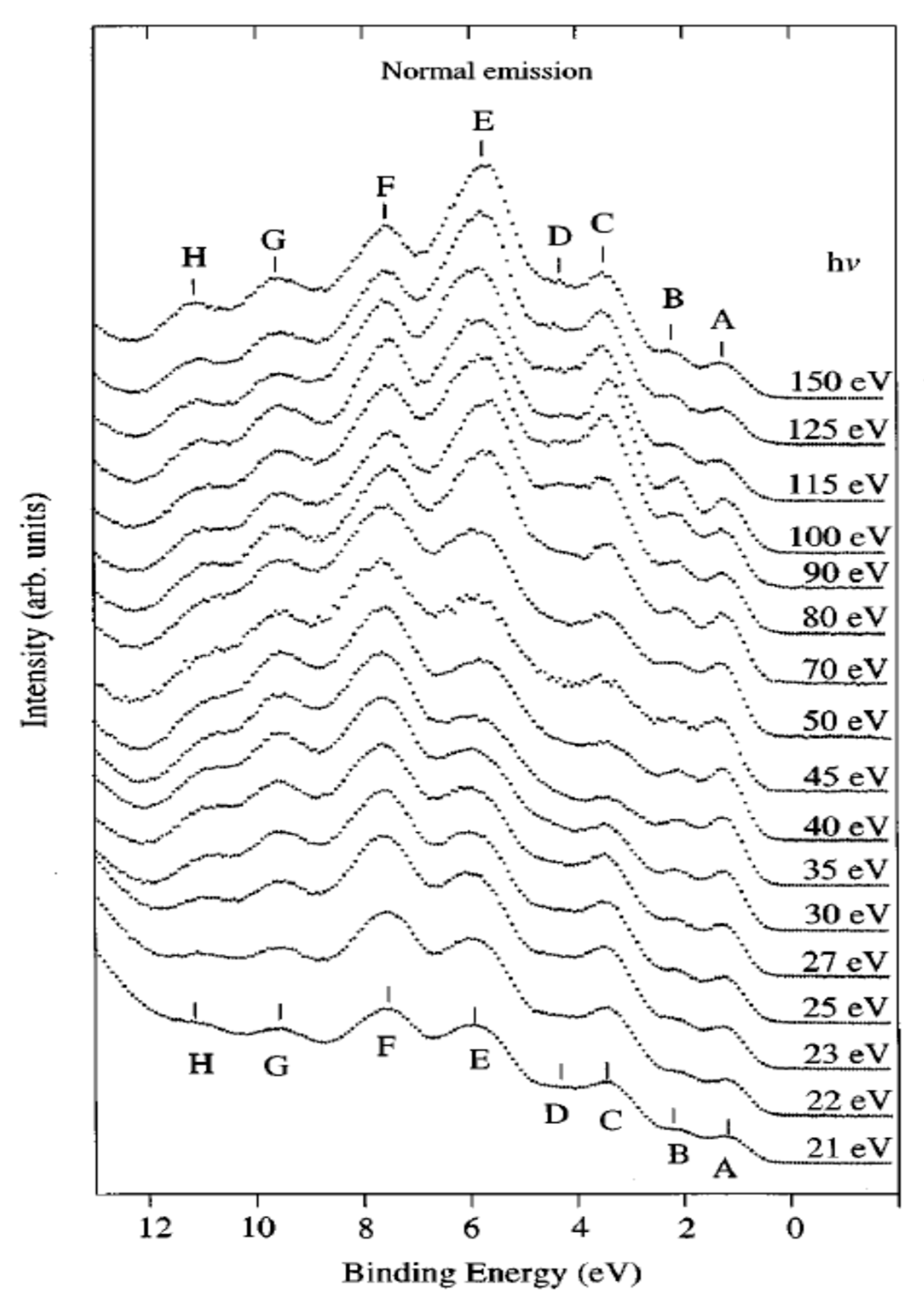
Crystals Free Full Text Angle Resolved Photoemission Study On The Band Structure Of Organic Single Crystals Html Coming Soon Movies Bollywood 2020 List The Binding Movie Wikipedia
Thus 136 ev is needed to ionize hydrogen to go from 136 ev to 0 or unbound an experimentally verified number.

Coming soon movies bollywood 2020 list the binding movie wikipedia. Since it takes energy to separate the electron and the proton once they are bound we call it mass defect. This mass defect is equal to the binding energy that binds the electron and the proton into a hydrogen atom. However unlike for hydrogen atom a closed form solution to the schroedinger equation for the many electron atoms like the lithium atom has not been found.
Ground state energy of hydrogen atom is equal to 136 ev. The kinetic energy is 136ev so when we add the two together we get the total energy to be 136ev. Mathematically mathmvr nh2pmath.
A hydrogen atom has less total energy then the separated constituents an electron and a proton. Okay from bohrs model the angular momentum of an electron in an orbit is an integral multiple of h2p. Given more energy the electron becomes unbound with some kinetic energy.
Kaliambos natural philosopher in new energy october 19 2015 lithium is an atom of the chemical element lithium with symbol li and atomic number 3. Since the ground state has. When an atom is in one of these states it does not radiate any energy but whenever there is a transition from one state to other energy is emitted or absorbed depending upon the nature of transition.
If the incident energy is large enough the electron can be knocked out of the atom leading to an ionization of atom. Let me try and answer your first question why the ground state of a h atom is 136 ev. If the binding energy of the electron in a hydrogen atom is 136 ev the energy required to remove the electron from the first excited state of li is a 1224 ev b 306 ev c 136 ev d 34 ev.
In fact we have to put in 136ev which is simply the ionisation energy of hydrogen. When an electron jumps from higher energy state to the lower energy state it emits radiations in form of photons or quanta. The total energy is negative because the electron is bound to the hydrogen atom and to remove the electron we have to put in energy.
However when an. An electron cant have an energy value half way between two energy levels in an atom. Whereas a free hydronium radical is a metastable species with a lifetime of 50 fs in its electronic ground state the hydrated hydronium radical can spontaneously decay into a h 3 o e ion pair in analogy to the na e pair in na doped water clusters which leads to the formation of a hydrated electron e aq 33 37 38.
Similarly a book cannot be placed half way between consecutive shelves.

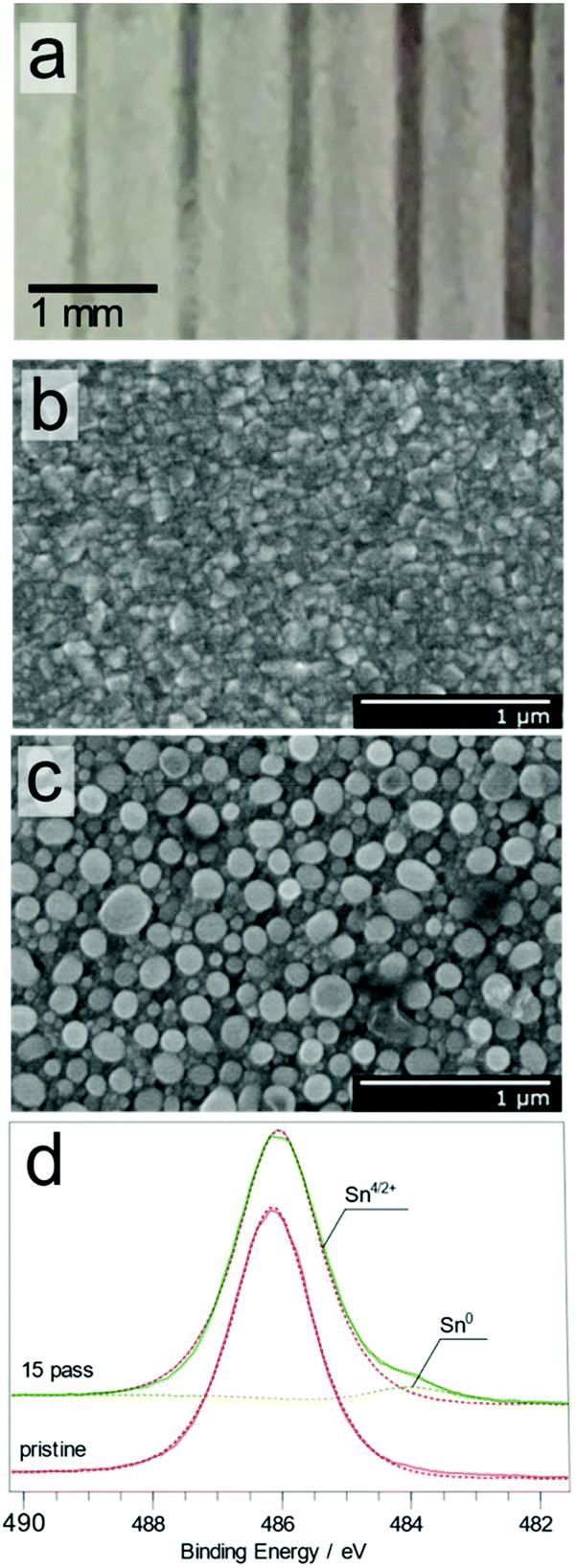
Pdf Effect Of Additives Ag And Rare Earth Elements Y And Sc On The Properties Of Hydrogen Sensors Based On Thin Sno2 Films During Long Term Testing Coming Soon Movies Bollywood 2020 List The Binding Movie Wikipedia